FDA OKs Marketing of Device to Reduce Opioid Withdrawal Symptoms (PharmacyTimes.com)

FDA clears Indiana-made device to ease opioid withdrawal (Indy Star)
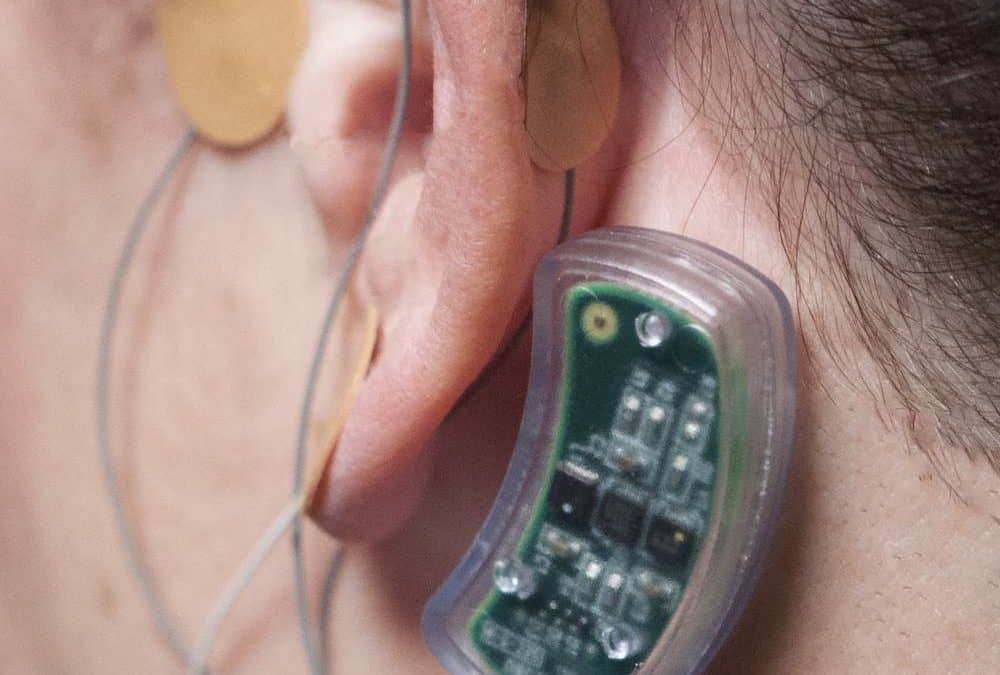
A Drugless Solution to Helping Opioid Addicts Recover

Could This New Device Offer Pain-Free Heroin and Opioid Withdrawal? (TheFix.com)
The groundbreaking behind-the-ear device is designed to help users in the initial stage of kicking narcotics.Innovative Health Solutions (IHS), a company based in Versailles, Indiana, has a bridge to sell you…
Recent Comments